Carbon is one of the
elements on the Earth. There are two types of carbon. They are Carbon 12 and
Carbon 14. Most of the carbon atoms are Carbon 12. It has a stable carbon atomic
structure and is non-radioactive.But Carbon 14 isn’t stable and has radioactive
carbon isotope.
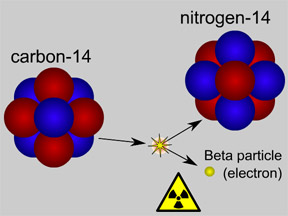
Carbon 14 is formed after
Nitrogen 14 is affected by cosmic rays and solar winds.
Both Carbon 12 and 14
existed in the atmosphere as carbon dioxide. They are absorbed by plants all
alike. Animals eat plants so they absorb Carbon 14 too.
When animals and plants are
alive, although Carbon 14 will change back to nitrogen, they continue to absorb
Carbon 14, so the ratio of both types of carbons in the bodies is more or less
the same as that in the atmosphere.
However, living things
won't be able to absorb Carbon 14 anymore after dying. Carbon 14 inside the
bodies will decrease. The longer they die, the less carbon 14 they have in their
bodies.
The radioactive half-life
of Carbon 14 is about 5,730 years, meaning that half of the Carbon 14 will be
converted back to nitrogen every 5,730 years. 5,730 years later, only 1/4 of the
original Carbon 14 will be left while 5,730 more years later, only 1/8 of the original Carbon 14 will be left.
The ratio of Carbon 14 and
12 in the atmosphere is about 1 to 10 billion.
Therefore, if a person
knows how much Carbon 14 a living thing had when it died, then he/she can
calculate how long it’s been dead.
The best equipment these
days, Accelerator Mass Spectrometer, can test the age of fossils as far as about
90,000 years ago.
However, there’s a
seriously wrong assumption in the test. Scientists who believe in evolution
assume that there’s no great mutation in the last billions of years and that
there’s no change in the elements in the atmosphere and cosmic rays. Therefore
they assume that the appearance and decline of Carbon 14 are already balanced.
They also think that the amount of Carbon 14 now and that billions of years
earlier are the same.
The ratio of carbon 14 will
be affected under certain situations. For instance, people began to burn lots of
coals and oil ever since the industrial revolution in 17th century. This
increases the amount of carbon in the atmosphere. But coals contain less Carbon
14 because they were located underground and were not affected by cosmic
rays.
Therefore, living things
existed after the industrial revolution have a lower ratio of Carbon 14 and
Carbon 12. If we don’t know this, then the age calculated by the equation will
be older than the object’s real age.
Another example is that the
amount of carbon 14 has increased after atomic bomb tests since 1940. Therefore,
the age calculated will be younger than the real one.
Moreover, the strength of
geomagnetic field will also affect the amount of Carbon 14. If it is great, it
will protect Carbon 14 from being affected by cosmic rays. It is confirmed
through tests of lava that our geomagnetic field has been decreasing in the last
thousands of years.
In addition, solar activity
and cosmic rays are more intense so the amount of Carbon 14 is greater than that
in the past. Therefore, testing samples thousands of years earlier will result in thousands
or even millions of years in error.
In fact, diamonds are
thought to be heated and under pressure underground for millions of years. The
Carbon 14 inside should be disappeared after millions of years but scientists
found that there is still Carbon 14 inside.
Contemporary scientist
discovered that the half-life of radioactive samples in the labs changes
instantly as the Earth is hit by the solar wind. Therefore, the assumption
saying that radioactive half-life is constant is completely broken.